1. Introduction
Vaccines have been a crucial basis of public health, significantly reducing the burden of infectious diseases globally. The COVID-19 pandemic has further accelerated the global deployment of vaccines to combat the virus’s spread. While these vaccines have been instrumental in controlling the pandemic, they have also highlighted various adverse effects, including immune-related adverse events (irAEs).[1,2] These irAEs have first gained attention with the use of immune checkpoint inhibitors (ICIs) in cancer treatment. ICIs have revolutionized cancer therapy by enhancing the immune system’s ability to fight tumors, yet they also increase the risk of inflammatory side effects.[3,4]
Cutaneous immune-related adverse events (cirAEs) affect a significant proportion of patients, sometimes necessitating the discontinuation of life-saving treatment.[4] These dermatological reactions can range from mild alopecia, urticaria, and angioedema to more complex conditions like life-threatening systemic anaphylaxis and drug reaction (or rash) with eosinophilia and systemic symptoms (DRESS syndrome).[5,6] The emergence of these reactions underscores the need for a deeper understanding of their mechanisms and implications, particularly as vaccines continue to play a pivotal role in global public health strategies.
Our research is driven by the need to enhance the safety and efficacy of vaccines for the global population. Previous research has largely focused on specific COVID-19 vaccines or limited patient populations, leaving gaps in our knowledge regarding the broader spectrum of these reactions across various vaccine types.[7,8] By systematically studying cirAEs using data from the global pharmacovigilance database, VigiBase, this study aims to fill these gaps. Furthermore, we provide a comprehensive analysis of cirAEs associated with a wide range of vaccines, offering a more detailed view of the potential dermatological impacts of vaccination.
2. Methods
The primary data source for this study is VigiBase, the world’s largest repository of individual case safety reports (ICSRs) maintained by the Uppsala Monitoring Centre (UMC) under the World Health Organization (WHO) International Drug Monitoring Program.[9] Established in 1968, VigiBase contains over 140 million adverse drug reaction reports from more than 170 countries.[10] Its extensive and reliable dataset enables comprehensive drug safety analyses and supports global pharmacovigilance efforts. Approval for using confidential and electronically processed patient data was obtained from the Institutional Review Boards of the UMC, a WHO Collaborating Centre. The requirement for informed consent was waived in this study, as VigiBase does not contain personal information.
Data on vaccine-associated cirAEs were collected from 1968 to 2024 and classified into 20 groups: (1) anthrax; (2) cholera; (3) diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b (DTap–IPV–Hib); (4) meningococcal; (5) pneumococcal; (6) tuberculosis; (7) typhoid; (8) encephalitis; (9) influenza; (10) hepatitis A; (11) hepatitis B; (12) measles, mumps, and rubella (MMR); (13) rotavirus diarrhea; (14) zoster; (15) papillomavirus; (16) COVID–19 mRNA; (17) Ad5-vectored COVID–19; (18) inactivated whole-virus COVID–19; (19) other viral vaccines (dengue virus, Ebola, enterovirus 71, monkeypox, respiratory syncytial virus, and smallpox vaccines); (20) others (brucellosis, leptospirosis, plague, and yellow fever vaccines). Adverse events were deduplicated globally using preferred terms from the Medical Dictionary for Regulatory Activities version 26.0. Under WHO causality assessment guidelines, only vaccines classified as ‘suspected’ were included in the analysis of disproportional associations with cirAEs.
This study meticulously recorded suspected cases of vaccine-associated cirAEs to ensure a comprehensive investigation. The study relied on the ICSRs submitted by various sources such as national pharmacovigilance centers, healthcare professionals, pharmaceutical companies, and patients. Reported data consists of various parts: patients demographics (i.e., age [0–11, 12–17, 18–44, 45–64, ≥65 years, and unknown] and sex), organizational data (i.e., reporting years [1968–1979, 1980–1989, 1990–1999, 2000–2009, 2010–2019, and 2020–2024], reporting region [African, America, Southeast Asia, Europe, Eastern Mediterranean, and Western Pacific], and reporter qualification [health professionals and non-health professionals]), vaccine information (i.e., vaccine class and suspected vaccine), and adverse drug reaction information (i.e., time to onset [TTO] of reaction and fatal outcomes [recovered, not-recovered, fatal, death, and unknown]).
This analysis used report and non-report groups to discover associations between vaccine types and the risk of cirAEs through disproportionality analysis. Two indicators are utilized in this analysis: the information component (IC) and the reporting odds ratio (ROR).[11] The IC was computed using the Bayesian method for the report–non-report analysis, comparing specific cirAEs to all other types of vaccines in Vigibase.[12,13] The statistical formula is as follows: log2((nobserved+0.5)/(nexpected+0.5)); nobserved = the number of actual adverse event case reports; nexpected = the number of expected adverse event case reports; nexpected = (ndrug * neffect)/ntotal; ndrug = the number of case reports for the drug with no regard of adverse events; neffect = the number of case reports for the adverse events with no regard of a drug; ntotal = total number of case reports.[12,14,15] The value IC0.25 means the lower limit of confidence interval (CI), and the positive IC0.25 value (IC0.25 >0.00) is considered to be significant. The ROR is a frequentist association measure obtained by analyzing the number of adverse events reported for a vaccine through a contingency table. A significant association between adverse events and the drug of interest is established when the ROR and its lower limit of the 95% CI exceed 1.00.[14,15] A two-sided p-value <0.05 was considered statistically significant. All analyses were performed using SAS (version 9.4; SAS Inc., Cary, NC, USA).
3. Results
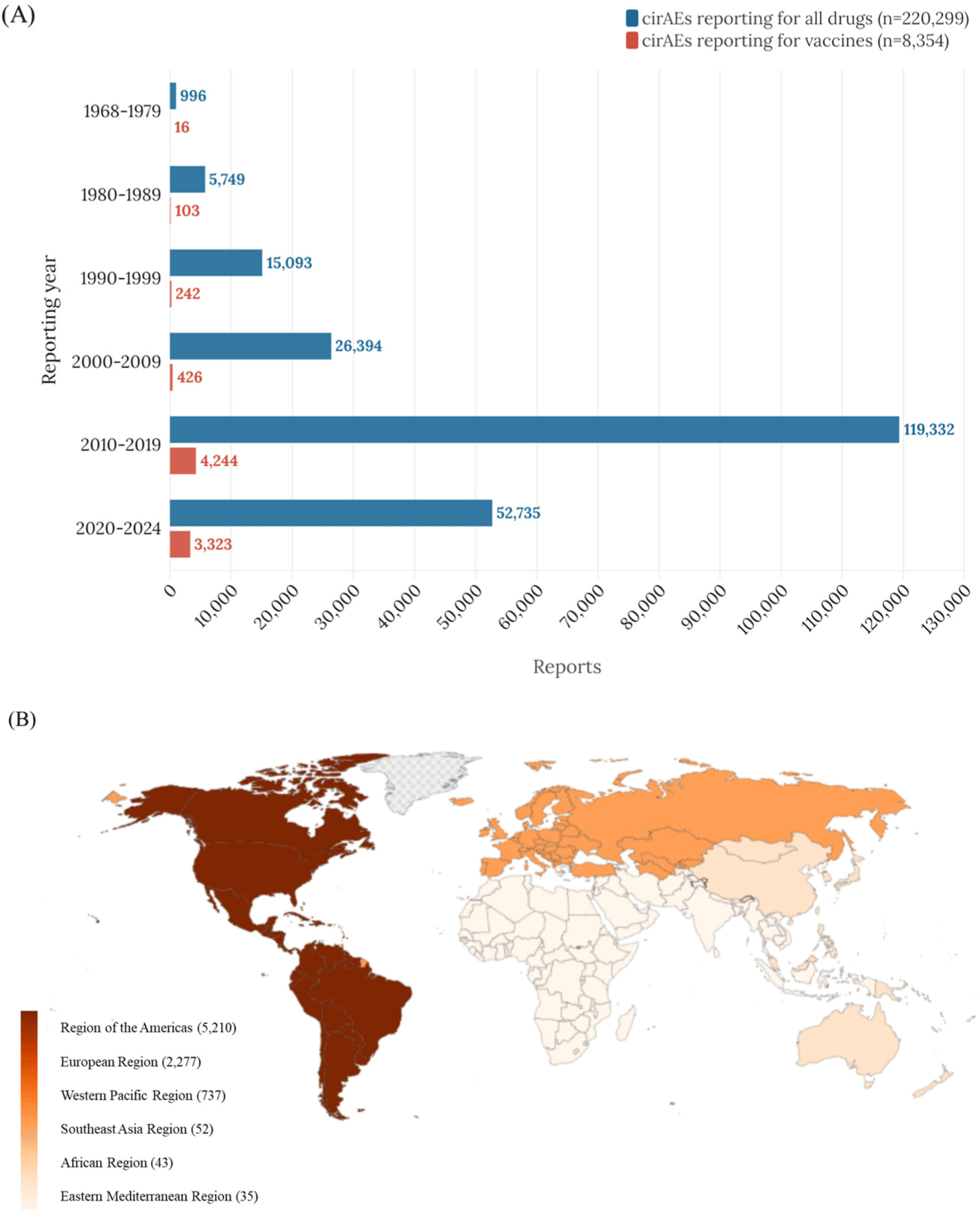
Among the 220,299 reports of all-cause cirAEs, 8,354 reports (male, n= 4,083 [48.87%]) were identified as vaccine-associated cirAEs in the VigiBase from 1968 to 2024 (Table 1). These reports were categorized into six geographic regions, with the highest number of cases reported in the Americas, followed by Europe, the Western Pacific, Southeast Asia, Africa, and the Eastern Mediterranean. Most cases occurred in the 0 to 11 years age group (46.65%). The highest number of adverse events were derived from COVID–19 mRNA vaccines (28.01%), followed by DTap-IPV-Hib vaccines (21.41%) and MMR vaccines (9.70%).
Abbreviation: cirAEs, cutaneous immune-related adverse events; DTaP-IPV-Hib, diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b; IQR, interquartile range; MMR, measles, mumps, and rubella; TTO, time to onset; WHO, World Health Organization.
As shown in Table 2, the analysis of vaccine-associated cirAEs revealed that most vaccines are significantly associated with cirAEs. Some viral vaccines (dengue virus, Ebola, enterovirus 71, monkeypox, respiratory syncytial virus, and smallpox vaccines) had the highest association with vaccine-associated cirAEs (ROR, 3.94 [95% CI, 3.30–4.71]; IC, 1.95 [IC0.25, 1.65]) followed by anthrax vaccines (ROR, 2.66 [95% CI, 1.96–3.62]; IC, 1.38 [IC0.25, 0.86]), hepatitis B vaccines (ROR, 2.24 [95% CI, 2.02–2.47]; IC, 1.15 [IC0.25, 0.99]), MMR vaccines (ROR, 2.15 [95% CI, 2.01–2.30]; IC, 1.10 [IC0.25, 0.98]), hepatitis A vaccines (ROR, 2.08 [95% CI, 1.82–2.39]; IC, 1.05 [IC0.25, 0.82]), typhoid vaccines (ROR, 1.84 [95% CI, 1.39–2.42]; IC, 0.86 [IC0.25, 0.39]), encephalitis vaccines (ROR, 1.50 [95% CI, 1.14–1.97]; IC, 0.58 [IC0.25, 0.11]), pneumococcal vaccines (ROR, 1.32 [95% CI, 1.22–1.43]; IC, 0.40 [IC0.25, 0.27]), DTap-IPV-Hib vaccines (ROR, 1.30 [95% CI, 1.25–1.37]; IC, 0.38 [IC0.25, 0.30]), and zoster vaccines (ROR, 1.14 [95% CI, 1.03–1.26]; IC, 0.19 [IC0.25, 0.02]).
Abbreviation: cirAEs, cutaneous immune-related adverse events; DTaP-IPV-Hib, diphtheria, tetanus toxoids, pertussis, polio, and Hemophilusinfluenza type b; IQR, interquartile range; MMR, measles, mumps, and rubella; TTO, time to onset; WHO, World Health Organization.
Bold style indicates when the value of IC0.25 is greater than 0.0 or the lower end of the ROR 95% CI is greater than 1.0. This means it is statistically significant.
When assessing the risk of adverse events across different age groups, significant values were observed within specific age groups. Among the 10 vaccine categories significantly associated with cirAEs, 6 categories represented the strongest association in the 0 to 11 years age group. For instance, typhoid vaccines (IC, 1.16 [IC0.25, 0.02]), encephalitis vaccines (IC, 0.99 [IC0.25, 0.38]), hepatitis A vaccines (IC, 0.77 [IC0.25, 0.47]), hepatitis B vaccines (IC, 0.91 [IC0.25 0.68]), MMR vaccines (IC, 0.45 [IC0.25, 0.33]), and zoster vaccines (IC, 0.63 [IC0.25, 0.43]) were most strongly associated with cirAEs in this age group. Typhoid vaccines (IC, 0.80 [IC0.25, 0.21]) and hepatitis B vaccines (IC 0.50 [IC0.25, 0.16]) also showed significant association in the 18 to 44 years age group. Additionally, anthrax vaccines (IC 1.07 [IC0.25, 0.52]) and some viral vaccines (IC 2.54 [IC0.25, 2.23]) were significantly associated with cirAEs exclusively in the 18 to 44 years age group. No significant differences were found based on sex.
A detailed description of the associations between different vaccine types and adverse reactions is represented in Table 3. Except for anthrax vaccines, COVID–19 vaccines, and other viral vaccine types, most adverse events have been reported in individuals under 18 years old. Fatal outcomes were rare, with the highest proportion occurring with Ad5-vectored COVID-19 vaccines (n = 4, 1.6%).
Abbreviation: DTaP-IPV-Hib, diphtheria, tetanus toxoids, pertussis, polio, and Hemophilus influenza type b; IQR, interquartile range; MMR, measles, mumps, and rubella; TTO, time to onset; WHO, World Health Organization.
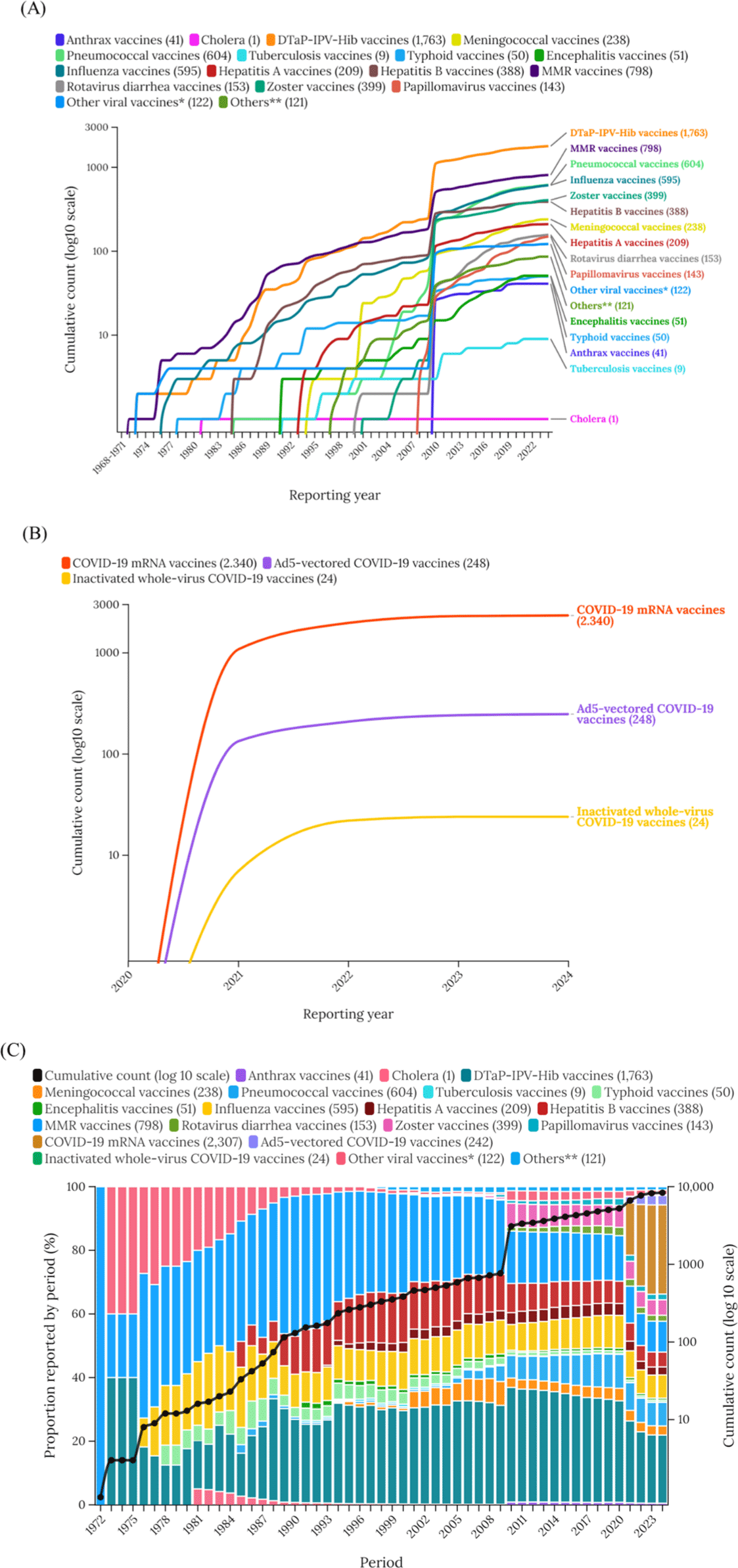
Fig. 1 and 2 show the cumulative vaccine-associated cirAEs reports. The number of reports steadily increased, with a notable surge in 2010, during which many adverse events were associated with the zoster virus vaccine. After 2020, COVID-19 vaccines (28.01%) accounted for most vaccine-associated cirAEs, whereas DTaP-IPV-Hib vaccines (21.41%) had been responsible for most reports before that period.
4. Discussion
Our study conducted a comprehensive and long-term investigation into the global burden of vaccine-associated cirAEs using the global pharmacovigilance database, VigiBase. The cumulative reports of cirAEs showed a steady increase over the entire period, with a significant rise around 2010, coinciding with a substantial increase in cases associated with the zoster virus vaccines. Following the emergency authorization of COVID–19 vaccines in 2020, these vaccines accounted for the greatest proportion of the adverse events, particularly COVID–19 mRNA vaccines. In terms of crude prevalence, only COVID–19 mRNA vaccines (28.01%) and DTap-IPV-Hib vaccines (21.41%) were over 20% among overall reports.
Overall, vaccine-associated cirAEs were not strongly associated with age and sex, although some vaccine types were associated with younger age groups. On an individual vaccine level, various viral vaccines—such as those for dengue, Ebola, enterovirus 71, monkeypox, respiratory syncytial virus, and smallpox—exhibited the highest association, followed by anthrax, hepatitis B, MMR, hepatitis A, typhoid, encephalitis, pneumococcal, DTaP-IPV-Hib, and zoster vaccines. The majority of these adverse events had only one day of TTO. Furthermore, more than half of the cases were reported to have recovered (50.47%), and 9.90% of the reports had not recovered. The fatality rate was 0.32%, with 0.04% of cases resulting in mortality.
Vaccine-associated cirAEs are an increasingly recognized concern, and understanding their underlying mechanisms is crucial for enhancing vaccine safety. Molecular mimicry is one of the primary mechanisms that cause cirAEs. Certain vaccine antigens share structural similarities with skin proteins, potentially triggering an autoimmune response.[16,17] For instance, the SARS-CoV-2 spike protein components used in mRNA vaccines theoretically mimic skin antigens, leading to conditions like bullous pemphigoid or psoriasis.[18] This mimicry causes the immune system to attack the foreign antigen and similar host tissues, resulting in cutaneous manifestations.
Another potential mechanism involves the role of immune system activation and cytokine release. When a vaccine is administered, it stimulates the immune system to produce a variety of cytokines, signaling molecules that help coordinate the immune response. In some cases, this response can become dysregulated, leading to an excessive release of cytokines, known as a cytokine storm.[19] This overproduction can cause inflammation and damage to the skin, resulting in various cutaneous manifestations. Understanding the balance between effective immune activation and excessive cytokine response is crucial for developing safer vaccines and managing adverse effects more effectively.
Pre-existing immune conditions and genetic predispositions can also play a significant role in the development of cirAEs. Individuals with a history of autoimmune skin conditions, such as lupus erythematosus or vitiligo, may be at higher risk for vaccine-associated cirAEs due to their already dysregulated immune systems. The vaccine or adjuvants used with the vaccine may act as a trigger, leading to the reactivation or worsening of these conditions or auto-immune/inflammatory syndrome induced by adjuvants (ASIA syndrome).[20] Additionally, genetic factors, such as tumor mutation burden and microsatellite instability, can affect the immune system’s recognition of self-antigens as foreign, thereby increasing the risk of autoimmune responses.[21] This is particularly relevant in vaccines that induce a strong cellular immune response, which may lead to tissue-specific autoimmunity.
Vaccine-associated cirAEs are a significant concern, especially as vaccination becomes more widespread. Understanding the implications of this study requires a thorough examination of the underlying immunological mechanisms, the identification of high-risk populations, and the development of strategies to mitigate these adverse effects.
Identifying populations at risk for cirAEs is crucial for tailoring vaccination strategies. Patients with pre-existing autoimmune conditions or a history of hypersensitivity reactions may be more susceptible to cirAEs due to their already dysregulated immune system.[22] This suggests that vaccination policies should include pre-vaccination screening protocols for individuals with a history of autoimmune diseases or severe allergic reactions, thereby preventing potential adverse outcomes.[23] Moreover, specific HLA genotypes have been linked to an increased risk of vaccine-associated autoimmunity, which may extend to the skin.[24] Therefore, incorporating genetic screening into vaccine administration guidelines for high-risk individuals could be beneficial.
Policy frameworks should prioritize post-vaccination surveillance systems capable of capturing and analyzing data on cirAEs to refine vaccine safety profiles and guide future recommendations.[25] Healthcare providers should be trained to recognize and manage cirAEs promptly to prevent escalation into more severe conditions. Additionally, patient education programs on the signs and symptoms of cirAEs and the importance of early intervention could help mitigate the severity of these events. Vaccine development strategies incorporating adjuvants that modulate immune responses more precisely might also be beneficial, minimizing the risk of cirAEs while maintaining immunogenicity.[26]
Vaccination strategies for young populations often differ due to the higher prevalence and increased severity of certain diseases in this age group. Vaccines such as DTaP, pneumococcal, MMR, hepatitis B, and others are part of the routine immunization schedules for young children in many countries due to the high burden of these diseases in early childhood. However, the implementation of these vaccination strategies necessitates a careful balancing act between the benefits of disease prevention and the risks of adverse events following immunization. For instance, while the MMR vaccine is crucial for preventing measles, mumps, and rubella—diseases with significant morbidity and mortality in children—the potential for cirAEs underscores the need for careful monitoring post-vaccination.
Despite these challenges, the benefits of vaccination generally outweigh the risks. For example, in the case of the COVID-19 vaccine, individuals who were vaccinated and subsequently infected with SARS-CoV-2 exhibited a lower risk of severe adverse events compared to those who were not vaccinated.[27] Thus, in regions where the incidence of vaccine-preventable diseases remains high, efforts should focus on maximizing vaccine coverage while implementing robust systems to monitor and manage adverse events, ensuring a balanced approach between the benefits of vaccination and the risks of adverse drug reactions.
This is the first study utilizing a long-term and extensive population-based global database to identify associations between various types of vaccines and cirAEs. VigiBase is a global pharmacovigilance database that aggregates reports from numerous countries, thus providing an extensive and diverse dataset. This comprehensive coverage allows for the identification of rare adverse events that might not be evident in smaller, localized studies.[28] The large volume of data enhances the statistical power of analyses, helping to detect patterns and signals that could indicate potential safety concerns. Additionally, VigiBase includes data from real-world settings, which means it consists of a wide variety of patient demographics and clinical scenarios such as sex, ethnicity, and the region of reports. This diversity can provide insights into how different populations might experience vaccine-associated cirAEs, potentially identifying specific risk factors or demographic groups that are more susceptible. Moreover, vaccine-associated cirAEs cases have increased for the last few years with the advent of COVID–19 vaccines.[1,2] Various kinds of vaccines were known to be risk factors inducing cirAEs, especially in the patients who were taking ICIs for cancer treatment.[29] However, few studies have investigated the effect of vaccines themselves, independent of concurrent ICI treatment, and even fewer have explored associations between different vaccine types and cirAEs. By including a broad range of vaccines in our analysis, this study provides a comprehensive overview of the impact of various vaccine types on the incidence of cirAEs.
However, this study has some limitations. One of the primary limitations of VigiBase is the potential for underreporting. As VigiBase is based on the spontaneous reporting system, not all adverse events are reported, and the likelihood of reporting can be influenced by factors such as the severity of the event, media coverage, and public awareness.[30] This can lead to an incomplete picture of the true incidence of cirAEs. Furthermore, the reports in VigiBase may lack detailed clinical information, such as the patient’s medical history, concurrent medications, and the precise clinical presentation of the cirAEs.[31] These limitations may impede the ability to conduct thorough analyses and fully understand the context of adverse events, particularly in relation to genetic susceptibility or predisposing immune conditions. Finally, establishing a direct causal relationship between vaccination and reported cirAEs can be challenging due to the observational nature of the data. Confounding factors and the absence of control groups make it difficult to differentiate between events caused by the vaccine and those that occur coincidentally.
While the use of VigiBase offers significant strengths, including a large and diverse dataset and real-world applicability, the limitations related to reporting biases, data completeness, and the lack of denominator data should be carefully considered when interpreting the present findings. These factors underline the importance of corroborating findings with other data sources, such as cohort studies or randomized controlled trials, to obtain a more accurate and reliable assessment of vaccine-associated cirAEs.
5. Conclusion
Public health has advanced significantly with the development and widespread use of vaccines, crucial for controlling infectious diseases. However, vaccines can lead to adverse events, such as cirAEs, raising safety concerns. This study quantified the global incidence of cirAEs and analyzed them based on demographic factors and vaccine types. We found no significant differences in cirAEs based on sex, but the incidence was higher among younger age groups. This underscores the need for vigilance, especially since many vaccines are administered to children for herd immunity. It is also important to consider the medical conditions of vaccinated individuals, as vaccines might worsen pre-existing conditions by triggering immune responses. Nevertheless, the benefits of vaccination are substantial, as the risk of severe or fatal outcomes following vaccination is generally low, with vaccinated individuals demonstrating a reduced risk of such adverse events. Future research should focus on understanding the mechanisms behind vaccine-associated cirAEs and developing strategies to manage these events.
