1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) presents a significant public health issue, as it is one of the most major causes of liver disease.[1] MASLD is caused by excessive accumulation of fat in the liver or progression of liver fibrosis, which can lead to fatal cirrhosis, hepatocellular carcinoma, and cardiovascular death.[2-4] MASLD occurs independently of alcohol consumption and is associated with an abnormality in insulin sensitivity and fatty acid uptake in the liver, and metabolic alterations mediated by an inflammatory mechanism.[5, 6] The global prevalence of MASLD is estimated to be 37.8% and is increasing at an rapid rate, placing an increasing burden on healthcare systems worldwide.[7]
Circadian rhythms, the biological patterns that regulate various physiological functions on an approximate 24-hour cycle, are profoundly influenced by the daily pattern of eating and fasting.[8, 9] Inappropriate eating patterns can disrupt these rhythms, leading to various health problems.[10-12] Recent studies show that eating rhythms are closely related to chronic diseases, and especially suggest that irregular eating habits or late meals can disrupt important metabolic processes such as lipid metabolism and insulin sensitivity, thereby increasing the risk of disease.[12-14]
Additionally, emerging evidence indicates that the timing of energy intake throughout the day significantly impacts an individual’s susceptibility to MASLD, with behaviors such as skipping breakfast, irregular meal patterns, and nighttime eating playing pivotal roles.[15] However, comprehensive assessments of meal frequency, timing, and time-specific energy intake distribution have been lacking, along with stratified analyses across demographic groups. Moreover, there was a shortage of extensive analyses targeting large populations.
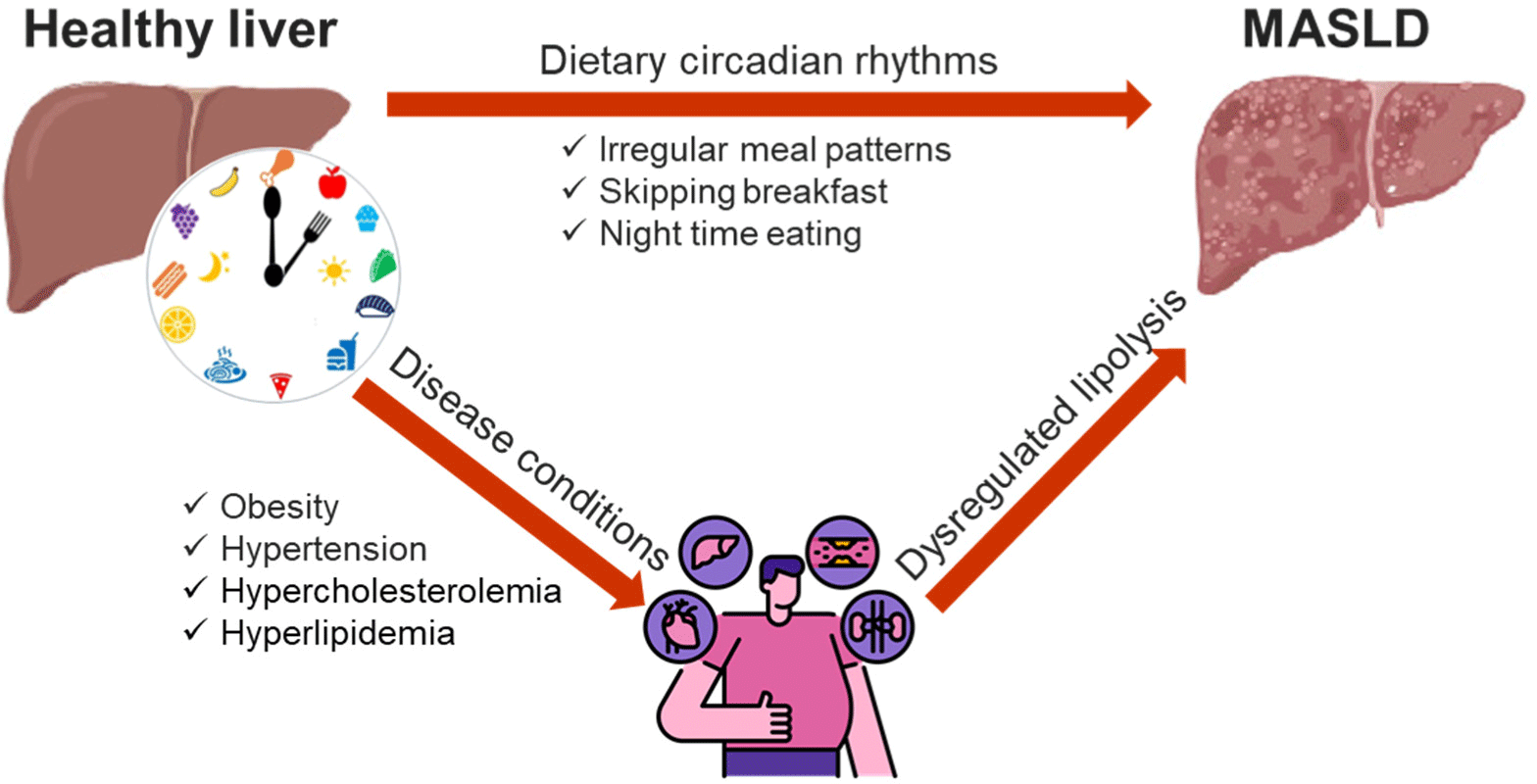
Therefore, we reviewed the potential association between meal frequency and timing and the risk of MASLD in adults (Fig. 1). These reviews suggest that adopting earlier daily eating patterns may be beneficial for MASLD prevention.
2. Comparison with previous studies
According to previous studies, skipping breakfast is associated with a number of adverse health outcomes, including MASLD, obesity, metabolic syndrome, and insulin resistance.[15, 16] Additionally, previous studies suggests that consuming a large number of calories during evening meals increases the risk of obesity and MASLD.[17, 18] Moreover, shifting the daily calorie intake to the morning has been suggested to be beneficial for MASLD.[18] These findings from prior research support the credibility of our findings.
Previous studies have indicated that irregular meal patterns, skipping breakfast, and night time eating may increase the risk of MASLD.[15] However, to our knowledge, there has been no comprehensive and stratified analysis conducted on meal frequency, timing, and time-specific energy intake distribution regarding the risk of MASLD. Furthermore, to our knowledge, no study has shown that meal patterns may have more significant influence on the risk of MASLD in groups with higher risk of MASLD. These findings will provide dietary guidelines to reduce the risk of MASLD and benefit public health.
3. Plausible underlying mechanisms
Our studies have shown that being a late eater, having the first meal after 9 AM, skipping breakfast, and consuming excessive calorie in the evening increase the risk of MASLD. These dietary habits can lead to circadian misalignment.[15, 19, 20] When circadian misalignment occurs, fat may accumulate in the liver, and inflammatory and pro-oxidative condition can be created, which can lead to non-alcoholic steatophepatits.[21] Consequently, circadian misalignment can increase the risk of MASLD.[22-24]
MASLD is generally more prevalent in males because estrogen has a protective effect against MASLD.[25-27] Therefore, even if women have unfavorable dietary habits, their risk of MASLD would likely be lower compared to males.
Obesity is a major risk factor for MASLD because it leads to fat accumulation in the liver, which triggers inflammatory process within the liver.[28, 29] When obesity-induced liver damage is compounded by circadian misalignment, liver damage may accelerates further. Therefore, in the obese group, the influence of MASLD due to dietary patterns would likely be more pronounced.
MASLD is also associated with hypertension[30, 31], hypercholesterolemia[32-34], and hyperlipidemia[35-37]. Similar to obesity, if circadian misalignment occurs in a state where the risk of MASLD is already elevated due to these underlying conditions, the risk of MASLD may increase even further.
To reduce the risk of MASLD, it is recommended to have breakfast before 9:00 AM and to shift overall eating time earlier in the day. These strategies may help align eating patterns with the body’s circadian rhythm, thereby improving lipid metabolism and insulin sensitivity. Additionally, prioritizing breakfast consumption and avoiding excessive calorie intake in the evening are advised to further lower the risk of MASLD. These recommendations may be particularly effective for reducing MASLD risk in males and individuals with pre-existing conditions associated with MASLD. By following these dietary guidelines, individuals can better manage their metabolic health and reduce the likelihood of developing MASLD.
4. Conclusion
Our study suggests that late meal patterns, skipping breakfast, and excessive calorie intake in the evening may increase the risk of metabolic dysfunction-associated steatotic liver disease (MASLD). These tendencies are particularly elevated among individuals with conditions such as obesity, hypertension, hypercholesterolemia, hyperlipidemia, and among males. Implementing time-based dietary approaches can be a useful strategy to improve the prognosis of adults with MASLD. By aligning meal timing with the body’s circadian rhythms, these strategies may help in managing and potentially reducing the risk of MASLD. However, our findings need to be validated through large-scale cohort studies to confirm their efficacy and generalizability. In summary, adopting an eating pattern that includes having breakfast before 9:00 AM, shifting overall eating times earlier in the day, and avoiding excessive evening calorie intake can be particularly beneficial. These measures are not only supportive of general metabolic health but also specifically target risk factors associated with MASLD.
