1. Introduction
Ranitidine is a histamine H2-receptor antagonist (H2 blocker) that is commonly used to reduce the production of stomach acid in patients with conditions such as acid indigestion and stomach ulcers.[1] On September 13, 2019, the United States Food and Drug Administration (USFDA) announced that preliminary tests found unacceptable levels of NDMA in ranitidine.[2, 3] On October 9, 2019, the Korea Food and Drug Administration (KFDA) reported that all ranitidine products in the Korea market were contaminated with unacceptable levels of N-nitrosodimethylamine (NDMA). Thus, regulatory authorities in South Korea, Singapore, Germany, Indonesia, Hong Kong, Ireland, France, and Canada recommended the recall of all ranitidine products.[2-4] Moreover, since the 1990s clinical studies have reported higher blood levels of NDMA in ranitidine users than non-users.[5, 6]
NDMA is a toxic semi-volatile organic chemical that occurs in various environments and at low levels including in certain foods, cosmetics, drinking water, tobacco smoke, and other manufactured products.[7-9] The International Agency for Research on Cancer (IARC) considers NDMA as “probably carcinogenic to humans” (Group 2A),[10] although this classification is not based on real-world conditions in humans. In July 2018, other researchers reported that some valsartan products (used to treat hypertension) were also contaminated with NDMA, leading to their withdrawal from the global market.[10]
We performed a nationwide propensity-matched cohort study in Korea to investigate the association between the use of ranitidine that was contaminated with NDMA with the risk of incident cancer and all-cause mortality. Our general aim was to provide real-world evidence for a relationship between the use of contaminated ranitidine with overall cancer risk and to assess the global public health impact of contaminated ranitidine.
2. Materials and Methods
Data were from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC). This is a large population-based cohort consisting of a representative sample (2.2%) of the Korean standard population that was established using systematic stratified random sampling and proportional allocation within each stratum (n = 1,000,000).[11, 12] The Korean NHIS provides mandatory and single-payer health insurance for all Korean citizens, and has records of nearly all personal data, health care records of inpatients and outpatients (including health care visits, prescriptions, disease diagnoses, and procedures), pharmaceutical visits, and the results of general health screening examinations (including questionnaires, blood tests, and physical examinations), and death records. All patient records used in this study were anonymized to ensure confidentiality. The study protocol was approved by the Institutional Review Board of Sejong University (SJU-HR-E-2019-006).
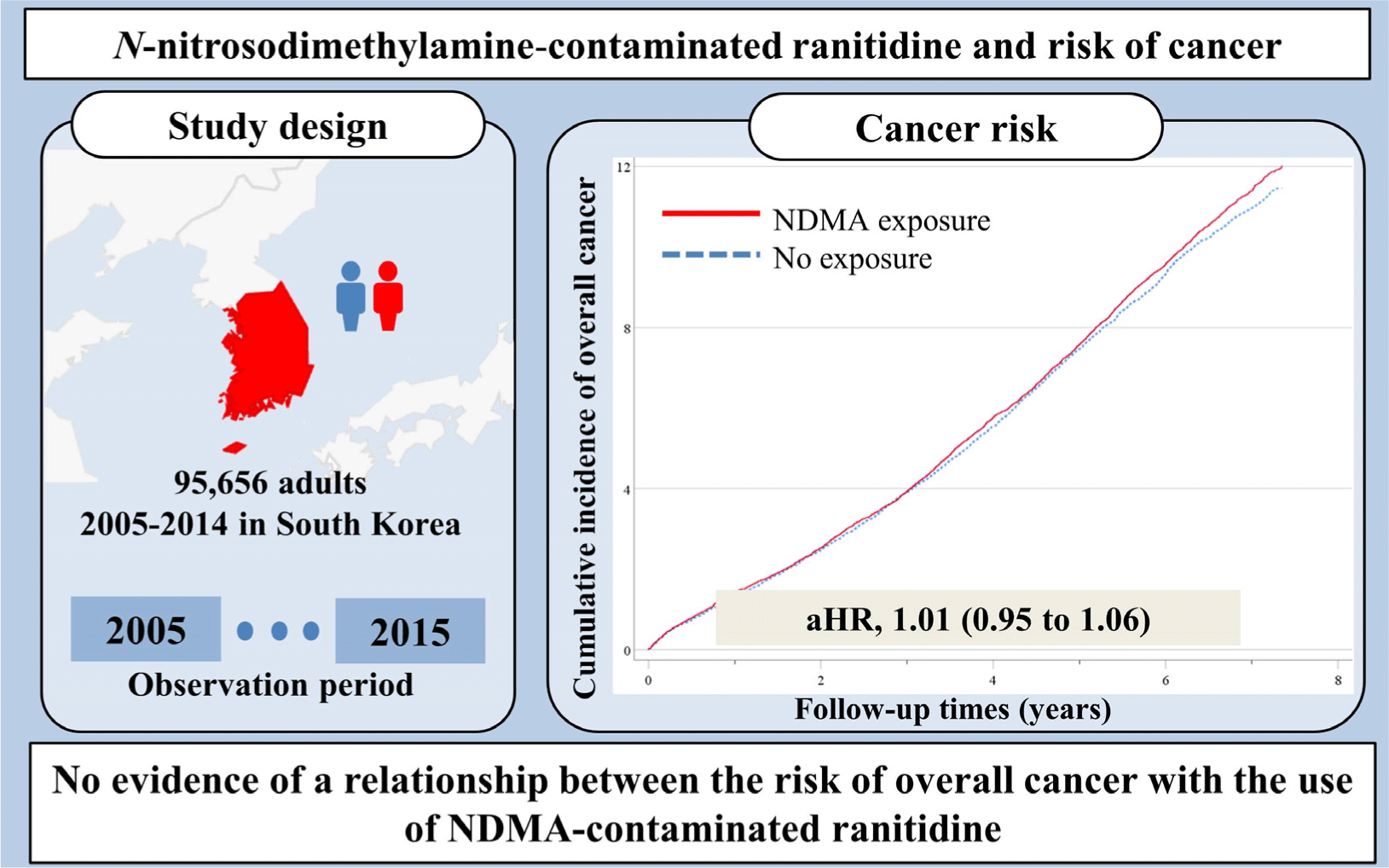
Eligible individuals were new users of H2 blockers (ranitidine, cimetidine, famotidine, nizatidine, roxatidine, or lafutidine) between 1 January 2005 and 31 December 2014. A “new user” was considered to be an individual who had no record of an H2 blocker prescription between 1 January 2002 and 31 December 2004. Users of H2 blockers contributed risk time of outcomes from 1 year after entering this cohort, thus a one-year lag-analysis was performed, in which patients with less than 1 year of follow-up were excluded. Among the initial 322,274 new users of H2 blockers, patients who were prescribed more than a 90 day supply during the 180 days after use of a new H2 blocker were also identified.[1] Patients were excluded if they had incomplete data; if they had a previous diagnosis of cancer based on a general health examination and/or previous assignment of an International Classification of Disease, 10th revision (ICD-10) code; if they were younger than 20 years; if their follow-up period was less than 1 year; or if they were new users of other H2 blockers but were previously users of ranitidine. Each cohort subject was followed after filling of the first prescription for an H2 blocker (individual index date), starting 1 year after cohort entry (one-year lag-analysis), and ending on 31 December 2015, development of incident cancer, or death. The final sample size was 95,656 (30,335 patients with “NDMA exposure” and 65,321 patients with “no exposure”).
KFDA reported that all ranitidine products in Korea market were contaminated with unacceptable levels of NDMA.[4] NDMA exposure was determined using a time-varying definition, and patients were categorized as having “NDMA exposure” (new users of contaminated ranitidine) or “no exposure” (new users of other H2 blockers and no use of contaminated ranitidine during the follow-up period). Patients were further stratified into tertiles by cumulative dose of contaminated ranitidine (<70,000, 70,000 to 129,999, or ≥130,000 mg) and cumulative prescription duration of contaminated ranitidine (<1, 1 to 2, or ≥2 years).
Incident cancer (primary outcome) was defined by use of an ICD-10 code of C at least 3 times within 1 year for an outpatient or 1 time for an inpatient.[11] C code designation is rigorously reviewed by the Korean NHIS to assure an accurate diagnoses, because it provides special insurance benefits to affected patients.[11] All-cause mortality (secondary outcome) was assessed using the national death index.
Covariates were recorded within 1 year before the first prescription of an H2 blocker. Sex, age, region of residence, and household income were collected from insurance data. Blood pressure and body mass index (BMI) were measured at the general health examination, and fasting blood glucose and total cholesterol were obtained from blood samples at the general health examination. History of diabetes mellitus, stroke, hypertension, smoking history, physical activity, and frequency of alcohol consumption were collected by self-reported questionnaires. The Charlson comorbidity index score (using ICD-10 codes) was calculated as reported previously.[11] The region of residence was classified as urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) or rural (Gyeonggi, Gangwon, Chungcheongbuk, Chungcheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju).[13] Use of selected classes of drugs within 180 days of the prescription before cohort entry were examined[14], including proton pump inhibitors, statins, systemic glucocorticoids, metformin, aspirin, and hormone replacement therapy.
The main analysis compared the risk of incident cancer (primary outcome) and all-cause death (secondary outcome) between the two groups in a propensity score-matched cohort. Propensity score matching was used to reduce potential confounding and to balance the baseline characteristics of the two groups.[15] Propensity scores were derived from the predicted probability of NDMA exposure vs. no exposure using a logistic regression model. The following covariates were considered potential confounders: age; sex; region of residence (rural or urban); alcohol consumption (<1, 1 to 2, 3 to 4, or ≥5 days per week); physical activity (0, 1 to 2, 3 to 4, 5 to 6, or 7 sessions per week); smoking history (never smoker, ex-smoker, or current smoker); household income (low, middle, and high); body mass index (<25, 25 to 30, or ≥30 kg/m2); systolic blood pressure, diastolic blood pressure, fasting blood glucose, serum total cholesterol (continuous variables); history of diabetes mellitus, stroke, or hypertension; Charlson comorbidity index (0 to 1, 2, or ≥3); and use of proton pump inhibitors, statin lipid-lowering drugs, systemic glucocorticoids, metformin, aspirin, or hormone replacement therapy. A “greedy nearest-neighbor” algorithm was used to match patients in the two groups in a 1:1 ratio.[16] Adequacy of matching was assessed by comparing propensity score distributions (Fig. S1 and S2) and standardized mean differences (SMDs); this approach is more meaningful that calculating P values of t tests because it is calculated as the population mean difference between groups and is scaled by the population standard deviation.[14] Statistical analyses were performed using SPSS version 25.0 (IBM Co.), R software version 3.1.1 (R Foundation), and SAS version 9.4 (SAS Institute Inc.). A two-sided P value below 0.05 was considered statistically significant.
Data were analyzed using COX proportional hazards regression models. Estimation of adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for the two groups in the matched cohort was performed with adjustment for covariates to reduce potential bias. A primary analysis (incident cancer), secondary analysis (all-cause death), analyses of cumulative exposure (dose and duration), and subgroup analyses of different types of incident cancer were performed. No violations of the proportional hazards assumption was identified based on analysis of Schoenfeld residuals and log-log survival plots.
Several sensitivity and supplementary analyses were performed to reduce the effect of bias. First all subjects were stratified by sex, age (<60 or ≥60 years at cohort entry), body mass index (normal [<25 kg/m2], overweight [25-30 kg/m2], or obese [≥30 kg/m2]), and smoking habit (ex/current smoker or never smoker). Second, the lag period was increased to 2 years or 3 years. Third, because Korea has a higher incidence of thyroid and stomach cancer than Western countries,[11] analysis was performed by excluding patients with these cancers. Fourth, because Valisure also detected NDMA in nizatidine (although at a level about 70 times less than in contaminated ranitidine)[17], analysis was performed by excluding patients who used nizatidine. Fifth, sensitivity analysis was performed to analyze the competing risks of incident cancer and all-cause death.[18] Finally, re-analysis of all the above sensitivity tests were performed using the full unmatched cohort.
3. Results
We identified 30,335 new users of contaminated ranitidine and 65,321 new users of other H2 blockers in the full unmatched cohort, and matched 27,792 new users of contaminated ranitidine and 27,792 new users of other H2 blockers in the propensity score-matched cohort (Fig. S3 and S4). The overall use of H2 blockers was stable during follow-up, however, the use of ranitidine in Korea steadily increased over 10 years (Fig. 1).

Table 1 shows the demographic and clinical baseline characteristics of the matched and unmatched cohorts. All SMD values were less than 0.02, suggesting the two groups had no major imbalances of baseline characteristics (Fig. S1 and S2). The median follow-up time in the matched cohort was 6.8 years (interquartile range [IQR], 3.9 to 9.2) for patients with NDMA exposure and 6.6 years (IQR, 4.0 to 8.7) for patients with no exposure.
During the follow-up period, there were 5,753 incident cancer events after propensity score matching, 2,840 among patients with no NDMA exposure (cancer incidence rate, 16.4 per 1,000 person years) and 2,913 among patients with NDMA exposure (cancer incidence rate, 17.0 per 1,000 person years) (Table 2 and Fig. 2). NDMA exposure was not related to the risk of incident cancer (aHR, 1.01; 95% CI, 0.95 to 1.06), and there was also no significant cumulative dose-response (P for trend = 0.429) or cumulative duration-response (P for trend = 0.549).
Abbreviations here: CI, confidence interval; H2 blocker, histamine H2 receptor antagonist; HR, hazard ratio; NDMA, N-nitrosodimethylamine; SD, standard deviation.
‡ Risk factors were adjusted for age; sex; frequency of alcohol consumption; region of residence (rural or urban); body mass index (<25, 25–30, or ≥30 kg/m2); systolic blood pressure, diastolic blood pressure, fasting blood glucose, and serum total cholesterol (continuous variables); history of diabetes mellitus, stroke, or hypertension; Charlson comorbidity index (0-1, 2, or ≥3), alcoholic drinks (<1, 1-2, 3-4, or ≥5 days per week); household income (low, middle, or high), smoking (never smoker, ex-smoker, or current smoker); physical activity (0, 1–2, 3–4, 5–6, or 7 sessions per week); and use of a proton pump inhibitor, statin, systemic glucocorticoid, metformin, aspirin, or hormone replacement therapy.

Secondary analysis indicated there were 3,691 all-cause deaths, 1,758 among patients who were users of contaminated ranitidine (mortality rate, 10.1 per 1,000 person years) and 1,933 among patients who were non-users (cancer incidence rate, 11.3 per 1,000 person years) (Table 3). NDMA exposure was not related to the risk of all-cause mortality (aHR, 1.04; 95% CI 0.97 to 1.11), and there was also no significant cumulative dose-response (P for trend = 0.424) or cumulative duration-response (P for trend = 0.117).
Abbreviations here: CI, confidence interval; H2 blocker, histamine H2 receptor antagonist; HR, hazard ratio; NDMA, N-nitrosodimethylamine; SD, standard deviation.
§ Risk factors were adjusted for age; sex; frequency of alcohol consumption; region of residence (rural or urban); body mass index (<25, 25–30, or ≥30 kg/m2); systolic blood pressure, diastolic blood pressure, fasting blood glucose, and serum total cholesterol (continuous variables); history of diabetes mellitus, stroke, or hypertension; Charlson comorbidity index (0-1, 2, or ≥3), alcoholic drinks (<1, 1-2, 3-4, or ≥5 days per week); household income (low, middle, or high), smoking (never smoker, ex-smoker, or current smoker); physical activity (0, 1–2, 3–4, 5–6, or 7 sessions per week); and use of a proton pump inhibitor, statin, systemic glucocorticoid, metformin, aspirin, or hormone replacement therapy.
Fig. 3 indicates the association of NDMA exposure with different types of incident cancers. These results indicate that NDMA exposure did not significantly alter the risk of incident cancers of the brain and nervous system, kidney and bladder, blood, lung, oral cavity and oesophagus, thyroid, sex-specific regions (breast, ovary, and prostate), stomach, pancreas and hepatobiliary system, and colorectum.

We also compared the aHRs for incident cancer of four different subgroups (Table S1). These results indicated that NDMA exposure was not associated with increased risk of incident cancer following stratification by sex (male: aHR, 0.97; 95% CI, 0.90 to 1.04; female: aHR, 1.05; 95% CI, 0.98 to 1.13), age (below 60 years: aHR, 1.05; 95% CI, 0.98 to 1.13: 60 years and above: aHR, 0.96; 95% CI, 0.89 to 1.04), body mass index (normal: aHR, 1.00; 95% CI, 0.94 to 1.07: overweight: aHR, 1.01; 95% CI, 0.92 to 1.10: obese: aHR, 1.14; 95% CI 0.85 to 1.53), and smoking status (never smoker: aHR, 1.01; 95% CI, 0.95 to 1.07; ex/current smoker: aHR, 1.01; 95% CI 0.91 to 1.11).
Sensitivity analyses indicated that NDMA exposure was not associated with incident cancer risk following use of two-year lag analysis, three-year lag analysis, exclusion of patients with thyroid and/or gastric cancer, exclusion of those who used nizatidine, and competing risk analysis (competing events: all-cause mortality and incident cancer) (Table S2). Finally, use of the same sensitivity tests in the full unmatched cohort also indicated that NDMA exposure was also not associated with risk of incident cancer or all-cause death (Table S3 to S4 and Fig. S4).
4. Discussion
We examined a Korean nationwide cohort to investigate the association of the use of contaminated ranitidine with incident cancer and all-cause mortality. Overall, our results indicated that users of contaminated ranitidine did not have an increased risk of incident cancer or all-cause mortality relative to users of other H2 blockers in the full unmatched cohort (n = 95,656) and in the propensity score-matched cohort (n = 55,584).
We also investigated the association of cancer risk with NDMA exposure after exclusion of nizatidine users. Valisure reported NDMA in this drug, although at levels 70-fold lower than those in contaminated ranitidine.[17] Moreover, we performed several sensitivity analyses in which subjects were stratified by sex, age, body mass index, smoking habit, thyroid and/or stomach cancer[11], and use of two year or three years lag periods. All the results of these sensitivity analyses were consistent with our primary results.
NDMA is a nitrosamine compound that is classified as a potential human carcinogen based on animal studies, although there is limited evidence of carcinogenicity in humans.[10] NDMA occurs in foods, cosmetics, drinking water, and tobacco smoke, but is not expected to cause harm when ingested in very low levels[7-9]. Nonetheless, there was significant public concern worldwide because it was recently detected in the antihypertensive drug valsartan[10] and the gastric acid inhibitor ranitidine[2, 3]. According to the KFDA guideline, the cutoff level for NDMA is 0.16 ppm, on the assumption that the lifetime maximum daily dose of ranitidine was 600 mg. All tested ranitidine products in the Korean market had NDMA levels of 2.78 to 53.50 ppm.[4] However, the Federal Institute for Drugs and Medical Devices (Germany), Therapeutic Goods Administration (Australia), and KFDA (Republic of Korea) concluded that, although taking a lifetime (25,550 days) of the maximum daily dose of ranitidine (600 mg) may be harmful, the short-term carcinogenic effect of contaminated ranitidine was not significant.[4]
The intestinal tract efficiently and rapidly absorbs most ingested NDMA (>90%)[19], and the liver then metabolizes it via α-hydroxylation or denitrosation;[20] α-hydroxylation may lead to the formation of methylating methyldiazonium (alkylates of biological macromolecules) and denitrosation may form methylamine and formaldehyde.[20] Specifically, the cytochrome P450-dependent mixed function oxidase system performs both of these metabolic conversions in the liver.[21] Finally, these pathways can lead to the formation of DNA adducts, such as N7-methyguanine and O6-methyguanine, which have strong mutagenicity and carcinogenicity because they induce direct mispairing.[22] As such, animal studies indicated that ingestion of NDMA is associated with increased carcinogenicity in the lungs, kidneys, gastrointestinal tract, and hepatobiliary tract.[7, 8, 20, 23]
Our results indicated that NDMA ingestion due to use of ranitidine was not associated with a significantly increased risk of incident cancer overall or of incident cancer at 10 specific sites. However, previous epidemiological studies reported that ingestion of NDMA was potentially associated with increased risk of cancer of the stomach[24], upper aerodigestive tract (larynx, esophagus, and oral cavity)[25], lung[26], colorectum[27, 28], and bladder[29]. The contrary findings of these other studies may be due to their small sample sizes, low-evidence study designs (cross-sectional or case-control), limited adjustment for confounders, or unexpected long-term exposure of NDMA.[24-29]
Previous research also reported the presence of NDMA in the antihypertensive valsartan.[10] However, the USFDA concluded that the high-temperature method used to detect NDMA generated high levels of NDMA from ranitidine, and that this method was therefore not suitable for measurement of NDMA in ranitidine.[3] Therefore the USFDA established a different low-temperature method for detection of NDMA in ranitidine and some products were still contaminated with unacceptable levels of NDMA.[3] However, previous studies reported that subjects who were given ranitidine had 6- to 12-fold higher levels of NDMA in their gastric juice[5, 6] and 430-fold higher levels in urine[6] compared with controls who did not receive ranitidine. Ranitidine contains amine moieties that can form N-nitrosamines in vitro, such as NDMA, when the pH is similar to that of the human gastric system[30]. This suggests there may be some in vivo synthesis of DNMA after ranitidine consumption[6]. In addition, the high metabolic conversion rate (>99.9%) and the low renal clearance (0.05%) of NDMA may provide a more meaningful measure of systemic NDMA exposure than its level in urine.[31] However, no epidemiological study has concluded that contaminated ranitidine was responsible for any cancer. Our research provides real-world evidence that there is no relationship between use of contaminated ranitidine with overall cancer.
Our study had several limitations that should be considered. First, we lacked information on important confounders related to exogenous and endogenous exposure to NDMA. The variables related to exogenous NDMA exposure include drinking water, foods (beer, fish, meats and cured meats, cereals, vegetables, and dairy products), cigarette smoke, and other factors.[32] The variables related to endogenous NDMA exposure include presence of a relevant pH in the gastric system, presence of nitrosating bacteria (i.e., Helicobacter pylori), plasma vitamin C level, and other factors.[32, 33] Although our assumption was that the NDMA dose correlated with the cumulative ranitidine dose, future studies should use individual data, consider exogenous and endogenous NDMA exposures, and measure the actual NDMA content of individual ranitidine tablets.
Another limitation is that we did not have data on cancer stage, severity, and pathology for our cohort. Also, all patients in this study were more than 20 years-old, and our results are therefore not applicable to pediatric populations. A final limitation is the short median follow-up period. Thus, our study only examined the short-term cancer risk after NDMA consumption, although our maximum follow-up period was more than 10 years. Thus, future studies of NDMA are needed to investigate the long-term cancer risk and to elucidate risk of cancer in pediatric populations.
A final limitation is that the source of NDMA in the contaminated ranitidine products is unknown.[3] However, studies since the 1990s have reported the presence of NDMA in users of ranitidine[5, 6], and there is an urgent need report our results because of the significant public concern of the many patients who have taken contaminated ranitidine during the past 10 years.
Despite these limitations, this is the first study to confirm no association of incident cancer or all-cause death with use of contaminated ranitidine in a general population of adults, based on analysis of a full unmatched cohort (n = 95,656) and of a propensity score-matched cohort (n = 55,584). The strengths of our study were that we used a nationwide cohort to reduce selection bias, we adjusted for multiple confounding factors related to cancer development, and we included blood sampling results and self-reported questionnaires. Second, we used a “new-user design” to reduce bias associated with the inclusion of prevalent users.[14, 34] Third, we used time varying exposure to eliminate immortal time bias and a one-year lag time to account for cancer latency.[14, 34] Finally, we compared two data sets to investigate the relationship between cancer and NDMA exposure: a full unmatched cohort and a propensity score-matched cohort. Our use of two cohort sets increases the reliability and generalizability of our findings, and provides strong evidence that use of contaminated ranitidine was not associated with risk of cancer.
Given the widespread use of ranitidine and the identification of NDMA contamination, physicians must consider whether they should remove it from their preferred prescription lists. Authorities in South Korea, Singapore, Germany, Indonesia, Hong Kong, Ireland, France, and Canada recommended the recall of all contaminated ranitidine medicines marketed in these countries.[4] Moreover, the USFDA recommends the use of alternative gastric acid inhibitors, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, because preliminary tests indicated no NDMA in these products.[2-4] Our results support the view that the health risks of exposure to NDMA due to consumption of contaminated ranitidine are low, and that physicians can reassure patients who have taken contaminated ranitidine that they face no increased risk of cancer. However, if a patient taking ranitidine wants to stop taking this drug, but still needs medication to treat acid indigestion, the physician should consider switching to another gastric acid inhibitor, as recommended by the USFDA.[2-4] Moreover, more stringent regulations regarding the manufacture of medical agents are needed to prevent global health hazards and to protect public health. Pharmaceutical companies and regulatory agencies worldwide should continue to scrutinize and monitor exposure to carcinogens, especially in medical agents.
5. Conclusion
Our results indicated no evidence of a relationship between the risk of overall cancer or mortality with the use of contaminated ranitidine in a Korean nationwide cohort. Multiple sensitivity analyses led to the same results. Although our results do not reassure keep using ranitidine, those suggest that clinicians should reassure their patients who used contaminated ranitidine that they face no markedly increased risk of incident cancer or mortality. Nonetheless, stringent global regulations on the manufacture of medical agents must be maintained to prevent public health problems worldwide, especially in commonly used pharmaceuticals.
Our results indicated no evidence of a relationship between the risk of overall cancer or mortality with the use of contaminated ranitidine in a Korean nationwide cohort.
Although our results do not reassure keep using ranitidine, those suggest that clinicians should reassure their patients who used contaminated ranitidine that they face no markedly increased risk of incident cancer or mortality.
Stringent global regulations on the manufacture of medical agents must be maintained to prevent public health problems worldwide, especially in commonly used pharmaceuticals.